Kelsey Chemistry
Average Atomic Mass Lab Activity, Calculating Mass Number of Isotopes
Average Atomic Mass Lab Activity, Calculating Mass Number of Isotopes
Couldn't load pickup availability
Have your students calculate the percent abundance, mass number of each isotope of your hypothetical element as well as the average atomic mass.
This average atomic mass lab activity is one of my student's favorites and is a great real life connection to average atomic mass.
WHO IS IT FOR?
- First year chemistry students learning the concept of average atomic mass, and how to calculate average atomic mass.
FEATURES
- Engaging
- Inquiry Activity
- Written conclusion to synthesize thoughts
- Post lab questions to help guide students through concept
WHAT MATERIALS ARE REQUIRED?
- Three different sizes of the same item: coins, dry beans, chocolates, pretzels, marshmallows etc.
- Balance (food safe if you're willing to let students snack on the isotopes)
WHAT’S INCLUDED
- Lab activity: pre and post lab questions and a written conclusion
- "How to Write a Chemistry Lab Report"
- Conclusion sentence starter prompt for differentiation
- Answer key with sample responses
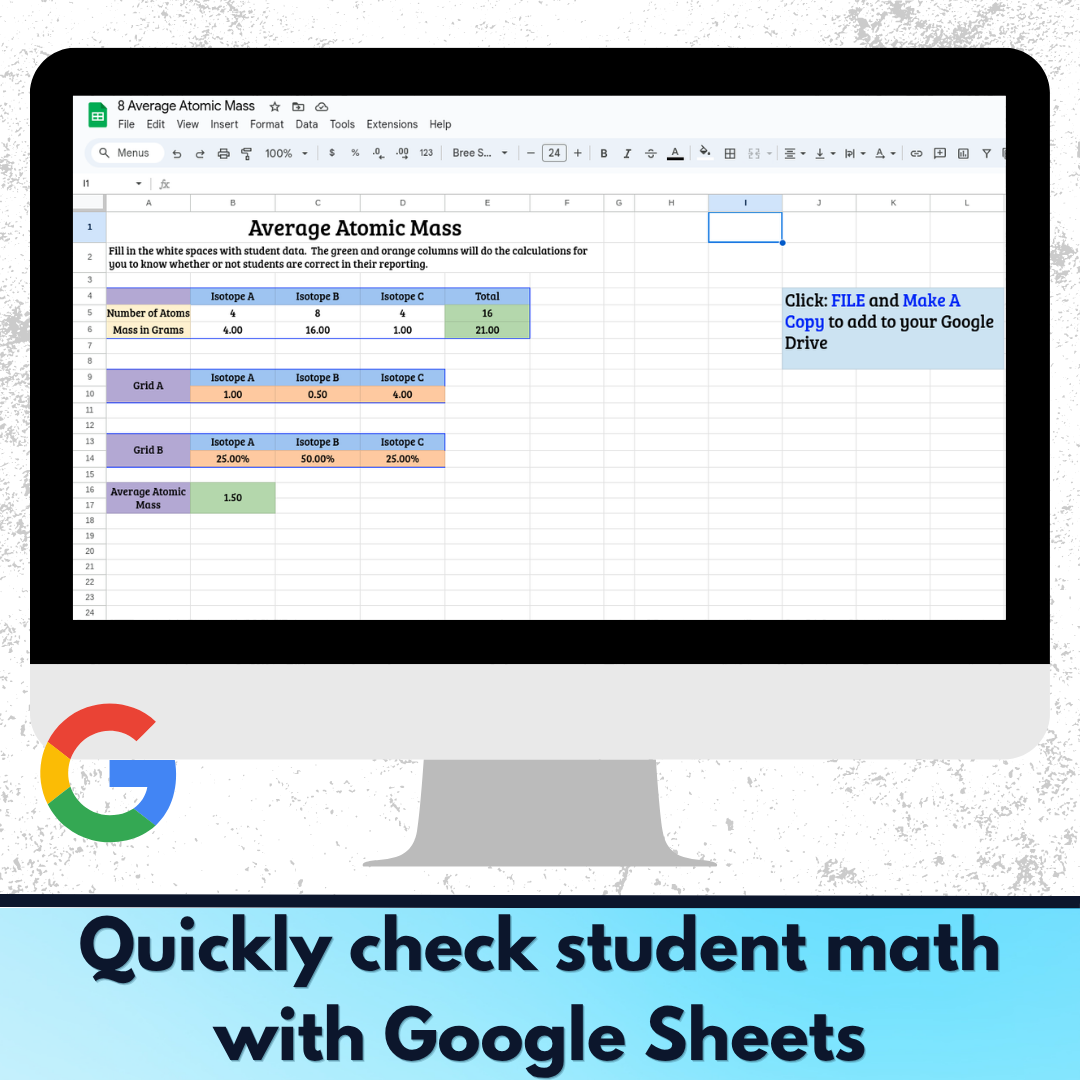
- Google Sheet calculator for quickly checking student calculations
- Teacher set up guide for guidance on how to create your isotope + Google Sheet with items you can use for isotopes with masses and mass numbers
INTERESTED IN MORE?
You can find this lab activity in my Full Year Chemistry Lab Book.
This product by Kelsey Reavy is copyrighted for single classroom only. This product may not be resold and can be copied for personal use within a classroom only. If you have questions, please email kelsey@kelseyreavy.com © Kelsey Reavy
Please Note: This is a digital product. No physical item will be shipped. After purchase, you’ll receive a link to download your files directly on the order confirmation page, and a download email will also be sent to you.
Share